First of all, what’s a Watt? Many of us are familiar with the term “Watt”, because it is used to describe things like how much electrical energy our lightbulbs, hair dryers, toasters and refrigerators use.
A “Watt” is not a unit of energy, however. It is a unit of power. To confuse us, the word “power” means something very specific in physics and engineering contexts; that same word “power”, however, means something quite different in the context of politics or sociology.
It’s important to disambiguate these uses of the word “power” in our minds when we talk about energy. (Ironically, however, the control of access to energy in our civilisations’ history necessarily involves political power, which is an interesting double-meaning of the word—this control point is the nexus where both meanings get entangled with each other.
Power in the physics sense simply means “Energy per unit of time.” A Watt is formally expressed as “one Joule of energy (used or generated) per second.” We use the letter “J” to denote Joules. As a shorthand we’ll also use prefixes like “kilo” and “mega” in front to indicate thousands or millions of Joules, e.g., kilo Joules (KJ) or mega Joules (MJ).
To convert from Watts (power) to energy (Joules) you just need to multiply by the length of time that you used a certain number of Watts. For example, a 100-Watt bulb lit for 60 seconds uses 100*60 = 6,000 Joules of energy.
If you look at the bill from your electric company, you’ll see “kiloWatt hours”. This is just another way of doing the same sort of math: a kiloWatt hour (kWh) is 1,000 Watts of power used continuously for one hour. It works out that one kWh is equal to 3,600,000 Joules (or 3.6 Mega Joules, or 3.6 MJ).
On a given day, a typical suburban house might use 30 kiloWatt-hours of electric energy, or about 1.4 kWh every hour. That’s about 5 million Joules per hour, or 5 MJ (mega Joules.)
So, now let’s talk about hydrogen, and tie this together.
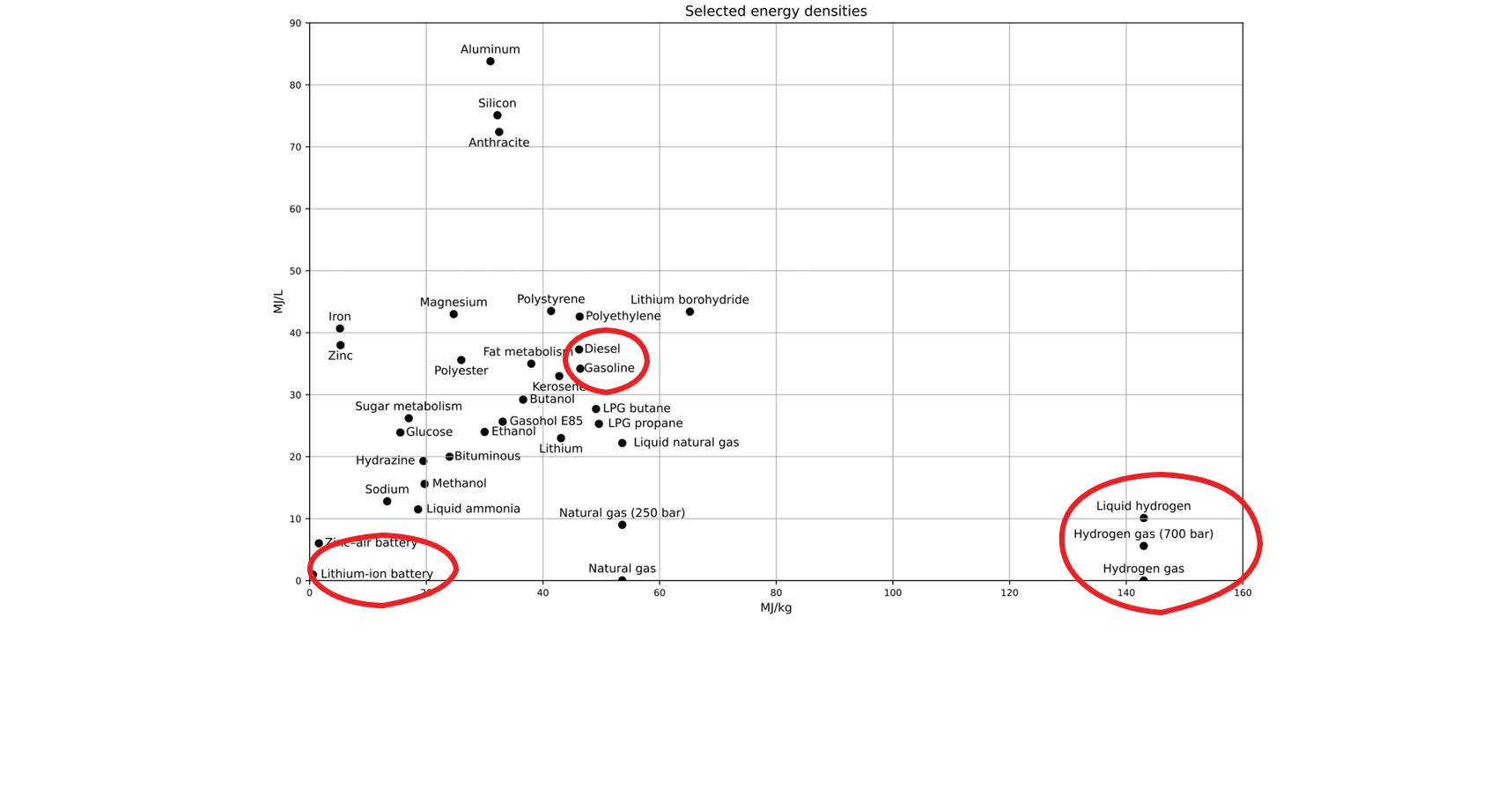
Here’s a chart that shows you where different substances stack up in terms of specific energy (Joules per kilogram) versus energy per volume (Joules per Litre.)
Because hydrogen is not very dense as a gas, it doesn’t offer as many Joules per Litre as other materials; but it still offers the most Joules per kilogram.
I have highlighted three areas: Lithium-ion batteries are what is used in electric cars; Diesel and gasoline is what is used in conventional internal combustion engines; and hydrogen is used for example in rocketry.
The further to the right on the chart, the more Mega Joules of energy are produced per kilogram of material. You can clearly see why rocket engines use pure hydrogen.
By the way, there are lots of reasons why electric cars are NOT the solution to the world’s energy problems: this chart hammers home one way that they really don’t stack up.
The obvious thing that most people (including Political Leaders) overlook with electric cars is this: how do you produce and distribute the electricity needed to charge the electric cars in the first place?
Right now, 90% of that energy is generated at a powerplant using fossil fuels like coal or natural gas, or possibly nuclear power plants.
When you use “the grid” to move that energy from the power plant to where you recharge your car, the lines carrying the power lose some of it along the way (efficiency loss—sometimes 5-15%.)
Using solar panels or wind turbines does NOT actually help either but that’s a complicated subject for another conversation about “energy return on energy invested”, which takes into account how much energy is needed in the first place just to produce the device (like the solar panel or wind tower) that is making your electricity for you. When one accounts for that properly, solar and wind power doesn’t look so appealing. Never mind that solar power doesn’t work at night, and wind power doesn’t work unless there’s a breeze.
Ironically the highest energy-return on energy-invested (EROEI) in the world right now is nuclear energy and we all know World Leaders views on that subject.
The best solution for energy production will always be to produce energy right at the place and right at the time it is needed, so that transmission (and storage) losses are eliminated.
OK, so now let’s circle back—to the topic of hydrogen.
We figured out above that a typical house needs about 5 MJ (mega Joules) of energy every hour. If we burned pure hydrogen in oxygen and somehow magically converted all of that energy to electricity 100% efficiently (impossible, by the way, but let’s go with it for now) we would need an amount of hydrogen found like this:
Take the 5 MJ of energy we need, divided by 141 MJ/kg (the specific energy, or energy of combustion of hydrogen) and the result comes to about 35 grams of hydrogen.
To power our home for an hour, we therefore need to burn at least 35 grams of hydrogen, in the ideal case. If we used natural gas instead, we’d need about 3 times as much, because the specific energy of natural gas is about 1/3 that of pure hydrogen—so roughly 100 grams (rounding out to nearest hundreds.)
All of this means that hydrogen — the most abundant substance in the entire Universe, which is also the cleanest and most abundant fuel for energy generation on our planet — could be used in a way such that perhaps 200 homes could be powered from the same amount of “fuel” that would today only power a single home.
If energy costs were to plummet by a factor of 200 – or even 20! — for all uses of energy worldwide, that would radically transform all world economies and make it possible for each of us to be largely self-sufficient.
All without any CO2 produced as a side effect. Furthermore, that energy could be produced on site at every home, where and when it is needed, eliminating the grid; water could be used as the source of hydrogen needed to power it; an electrolyser could be used to power your car, or at least, your car could be charged from your home’s own energy source, freeing it from the grid; and all of this would open the door to a kind of energy independence for individual people around the world that has never been dreamed of before in world history.